A nickel-cadmium battery (Ni-Cd or Ni-Cad) is a rechargeable battery used in portable computers, rigs, video cameras, and other small battery-powered devices that require uniform discharge. Ni-Cds use electrodes made from nickel oxide hydroxide, cadmium metal, and potassium hydroxide alkaline electrolyte.
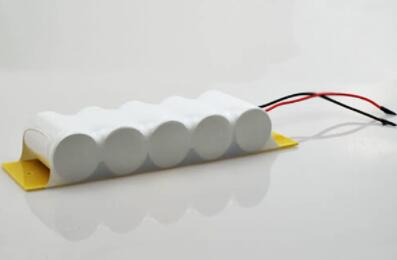
Two or more Ni-Cd battery cells combined to form a battery pack. Because they are typically similar in size to primary cells (non-rechargeable batteries), Ni-Cds may have lower terminal voltages and smaller ampere-hour capacities. However, unlike primary cells, Ni-Cds provide a nearly constant terminal voltage during discharge, which can result in an almost undetectably low charge. During discharge, Ni-Cds convert chemical energy into electrical energy. During charging, Ni-Cds convert electrical energy back into chemical energy.
✳Withstand long periods of deep discharge
✳More charge/discharge cycles than other rechargeable batteries to extend battery life
✳Higher energy density, lighter and more compact than lead-acid batteries Ni-Cd is preferred when size and weight are critical factors, such as in aircraft
✳Lower self-discharge rate than nickel-metal hydride (Ni-MH) batteries (20% per month vs. 30% per month)
Ni-Cd batteries are highly toxic. In addition, nickel and cadmium are expensive metals.
Unlike lead-acid batteries, Ni-Cd batteries can overheat, go into thermal runaway mode and self-destruct when charged with a generator - even in an overcurrent cutoff system. However, Ni-Cd battery packs are usually equipped with an internal thermal charger cut-off device that signals if the battery heats up and/or reaches its maximum voltage.

 Ni-MH Battery C4700mAh 3.6V
Ni-MH Battery C4700mAh 3.6V Nickel Cadmium Nicd Battery Pack SC1800mAh 3.6V
Nickel Cadmium Nicd Battery Pack SC1800mAh 3.6V Ni-Cd Battery Pack D4000mAh 3.6V
Ni-Cd Battery Pack D4000mAh 3.6V Ni-Cd Battery Pack C2500mAh 3.6V
Ni-Cd Battery Pack C2500mAh 3.6V NICAD Battery Pack AA900mAh 3.6V
NICAD Battery Pack AA900mAh 3.6V LiFePO4 IFR18650 1600mAh 3.2V
LiFePO4 IFR18650 1600mAh 3.2V LiFePO4 IFR18650 1600mAh 6.4V
LiFePO4 IFR18650 1600mAh 6.4V Ni-MH Battery C4000mAh 3.6V
Ni-MH Battery C4000mAh 3.6V E-bike Battery 48V 10Ah JL-1
E-bike Battery 48V 10Ah JL-1 E-bike battery 48V 10Ah Qing Tian
E-bike battery 48V 10Ah Qing Tian