Nickel-cadmium battery is a source for DC voltage. Due to its properties and advantages, it is taking over lead acid-based batteries and gaining popularity in recent times.
It’s a device that produces, DC voltage based on the chemical reaction between the substances involved. In a nickel-cadmium battery, the redox material is used as a base, and around it, the layer of nickel and a separator are used. The nickel-cadmium cell voltage is around 1.2 V. When connected in series generally 3 to 4 cells are packed together to get an output of 3.6 to 4.8 V.
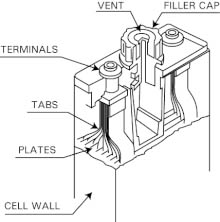
Nickel-Cadmium Battery Design
The operating principle of a nickel-cadmium battery is the same as other batteries. To improve efficiency, nickel and cadmium are used. A battery is the source of DC voltage, hence it must consist of two potential points i.e positive and negative or also called anode and cathode. In a nickel-cadmium battery, first, a layer of nickel oxide NiO2 is kept around the redox.
This layer of nickel oxide acts as a cathode layer. Above the nickel oxide layer, a layer of KaOH is kept, which acts as a separator. It must be noted that this separator layer must be soaked in water or moist. Its purpose is to provide required OH negative ions, for the chemical reaction. Above the separator layer, cadmium is placed. The cadmium layer acts as the anode for the nickel-cadmium battery.
The nickel acts as a positive electrode collector and the cadmium layer acts as a negative layer collector. The separator layer between the two layers is made up of KOH or NaOH. Its purpose is to provide OH ions. Apart from these, it also consists of a safety valve, sealing plate, insulation ring, insulation gasket, and an outer case.
The purpose of the insulator ring is to provide insulation between the two layers. The insulator gasket is the place where the insulation ring is kept nearby. The separator layer is connected to this ring. The outer case is to provide protection to the inner layers from external factors such as damages and mishandling of the battery. It must be noted that, due to chemical reactions taking place within the batter, it always hazardous to work with the battery.
The case of the battery is never opened, as all the layers are exposed and it may cause harm to the person using. Similarly, when not in use, it is recommended to remove the battery out of the device.
The chemical equations representing the chemical reaction can be given as
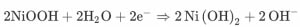
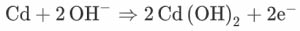
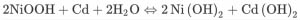
The first equation represents the reaction between the cathode layer nickel and the separator. It gives an output of Nickel oxide OH ions. The need for the separator layer as mentioned before is the provide the OH ions required for the chemical reaction. For the provision of H20, the separator layer is soaked with water for the initial reaction. Later H2O is obtained as one of the byproducts.
On the anode side, the cadmium layer is also combined with OH ions which are obtained from the separators layer. This results in cadmium oxide and electrons. It may be noted that the electrons in both the equations get canceled. Also, OH ions get canceled. The reminder equation is given by the third equation, where nickel is combined with cadmium and water. It results in nickel oxide and cadmium oxide.
The temperature range for nickel battery is 0 to 45-degree centigrade during charging and -20 to 65 degrees centigrade during discharging. Beyond this temperature range, the battery fails to operate and even chances of explosion exist.
Nickel-cadmium battery is very toxic to the human body. Cadmium is a heavy metal posing several risks to the human body. Cadmium even has a physiological effect on the system. The average presence of cadmium in the human body is approximately 1 microgram per liter. It has a direct effect on the digestive system. Similarly, nickel is also poisonous to the human respiratory system.
In general, each voltage for a Nickel-cadmium battery would be approximately 1.2 V. Number of cells are connected in series or parallel to get the required voltage. Apart from the voltage, its specific energy is around 50-60 Wh per Kg. This is moderately high that nickel-iron, but relatively less than nickel-zinc and nickel-metal hydride batteries.
The specific power is 200 W per kg. This is moderately high than nickel-iron but relatively less than nickel-zinc and nickel-metal hydride batteries. For nickel-metal batteries, it is around 170-1000. For nickel-iron batteries, it is around 100. The energy efficiency is around 70-75%. This is moderately high than nickel-iron but relatively less than nickel-zinc and nickel-metal hydride batteries. For nickel-metal batteries, it is around 70-80 %. For nickel-iron batteries, it is around 60-70%.
Constructional, the nickel-cadmium battery is the same as lead acid-based batteries. It consists of three fundamental layers. The first one is a nickel layer, then the separator layer, and the cadmium layer. The nickel acts as a positive electrode collector and the cadmium layer acts as a negative layer collector.
The separator layer between the two layers is made up of KOH or NaOH. Its purpose is to provide OH ions. Apart from these, it also consists of a safety valve, sealing plate, insulation ring, insulation gasket, and an outer case. The purpose of the insulator ring is to provide insulation between the two layers. The insulator gasket is the place where the insulation ring is kept nearby. The separator layer is connected to this ring.
The outer case is to provide protection to the inner layers from external factors such as damages and mishandling of the battery. It must be noted that, due to chemical reactions taking place within the batter, it always hazardous to work with the battery. The layers along with the separator layer form the required chemical reaction and form the potential difference.
The working of the nickel-cadmium battery is based on the chemical reaction taking place between the layers. The battery which is a source of DC voltage consists of two ports i.e. anode and cathode. While making the battery, first the cadmium layer is kept on the redox. The cadmium layer acts as the cathode terminal. Cadmium is one of the heavy material and has good conducting properties. Above the cadmium layer, separator layers are kept.
The purpose of the separator layer is to provide required OH ions for the chemical reaction. The OH ions are required for the reaction between the cathode layer nickel and the separator. It gives an output of Nickel oxide OH ions. The need for the separator layer as mentioned before is the provide the OH ions required for the chemical reaction. For the provision of H20, the separator layer is soaked with water for the initial reaction.
Later H2O is obtained as one of the byproducts. On the anode side, the cadmium layer is also combined with OH ions which are obtained from the separators layer. This results in cadmium oxide and electrons. It may be noted that the electrons in both the equations get canceled. Also, OH ions get canceled. The reminder equation is given by the third equation, where nickel is combined with cadmium and water. It results in nickel oxide and cadmium oxide. The chemical reaction is followed by the flow of electrons which causes the potential difference across two terminals.
Nickel-cadmium battery classification is only done based on size and available voltage. Based on size it may be of AAA, AA, A, Cs, C, D, or F size. All these sizes come with different output voltage specifications. Some of them are cylindrical pipe-shaped and some of them are in a rectangular box-shaped outer case.
The advantages of Nickel Cadmium Battery are
● Delivers high current output
● It tolerates overcharging
● It withstands up to 500 cycles of charging
The disadvantages of Nickel Cadmium Battery are
● Cadmium is not an eco-friendly material
● Less tolerance towards temperature as compared to other batteries.
It has various applications like toys, small DC motors, calculators, fans, computers, etc.
Hence we have seen the applications, working, and details of nickel-cadmium battery. It is must be seen what are other material which can be combined with nickel since cadmium has hazardous effects.

 Ni-MH Battery C4700mAh 3.6V
Ni-MH Battery C4700mAh 3.6V Nickel Cadmium Nicd Battery Pack SC1800mAh 3.6V
Nickel Cadmium Nicd Battery Pack SC1800mAh 3.6V Ni-Cd Battery Pack D4000mAh 3.6V
Ni-Cd Battery Pack D4000mAh 3.6V Ni-Cd Battery Pack C2500mAh 3.6V
Ni-Cd Battery Pack C2500mAh 3.6V NICAD Battery Pack AA900mAh 3.6V
NICAD Battery Pack AA900mAh 3.6V LiFePO4 IFR18650 1600mAh 3.2V
LiFePO4 IFR18650 1600mAh 3.2V LiFePO4 IFR18650 1600mAh 6.4V
LiFePO4 IFR18650 1600mAh 6.4V Ni-MH Battery C4000mAh 3.6V
Ni-MH Battery C4000mAh 3.6V E-bike Battery 48V 10Ah JL-1
E-bike Battery 48V 10Ah JL-1 E-bike battery 48V 10Ah Qing Tian
E-bike battery 48V 10Ah Qing Tian